Bussiness
US approves schizophrenia drug with new mode of action
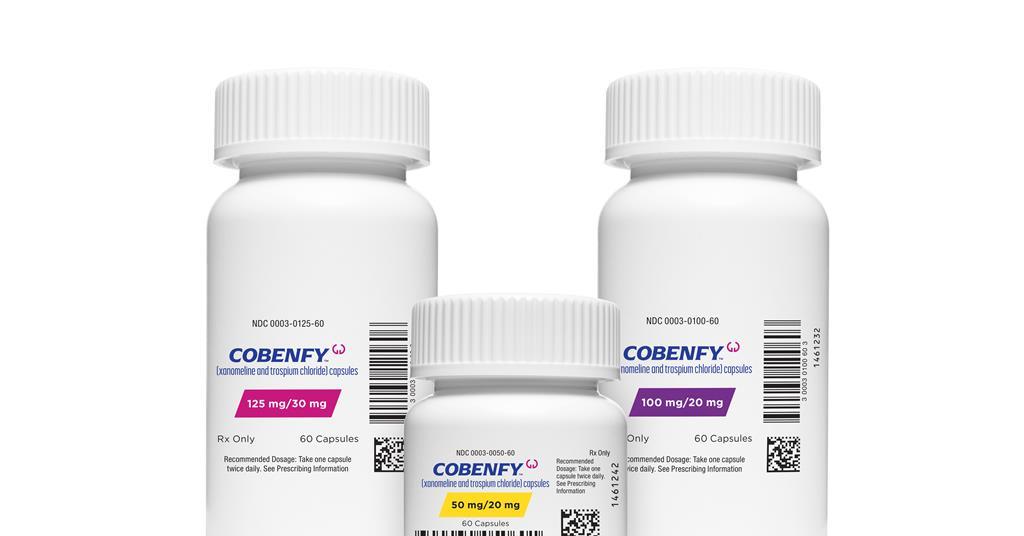
The US Food and Drug Administration (FDA) has approved a drug for schizophrenia that is exciting the field with an entirely new mechanism. The twice daily oral pill, Cobenfy, is the first to target cholinergic receptors rather than dopamine receptors.
Schizophrenia can be a disabling disease that induces psychotic symptoms such as hallucinations, trouble controlling one’s thoughts and paranoia, as well as difficulties with social interactions and motivation.
Cobenfy combines xanomeline and trospium and was shown in two phase 3 trials (Emergent-2 and Emergent-3) to be safe and effective in adults with schizophrenia. The drug was obtained by Bristol Myers Squibb when it bought Karuna Therapeutics for $14 billion (£11 billion) in 2023 to strengthen its neuroscience portfolio.
Schizophrenia is linked to imbalances in the neurotransmitters acetylcholine and dopamine in the brain. Existing schizophrenia treatments act on dopamine in the brain but have shortcomings.
‘They help with positive symptoms like hallucinations and delusions but do not work well for negative symptoms like feeling unmotivated and cognitive symptoms such as problems with memory and attention,’ says Robert McCutcheon, a psychiatrist at the University of Oxford, UK.
Xanomeline offers a new approach because it selectively targets two muscarinic acetylcholine receptor subtypes (M1 and M4) in the central nervous system. However, muscarinic receptors are present throughout the body, so targeting them can cause side effects such as nausea and gastrointestinal upset.
There was initial interest in xanomeline as a potential treatment for Alzheimer’s disease in the 1990s, but side effects proved off-putting. PureTech Health licensed xanomeline from Eli Lilly and combined it with trospium. Karuna was later founded to develop the treatment.
Potentially this is the most important antipsychotic developed in the last 30+ years
The addition of trospium, which is approved on its own as a treatment for urinary incontinence, was to reduce side effects by blocking muscarinic receptors outside of the brain. Trospium does not cross the blood-brain barrier, so receptors there remain unblocked.
‘This means that the beneficial effects in the brain can occur while the side effects in the rest of the body can to some extent be minimised,’ noted McCutcheon. Nevertheless, the FDA’s list of common side effects for Cobenfy includes nausea, indigestion, vomiting and constipation.
‘Potentially this is the most important antipsychotic developed in the last 30+ years,’ noted Robin Murray, psychiatrist at King’s College London, UK. ‘It’s the first not to work by simply blocking dopamine D2 receptors. It has different, possibly fewer side effects, and may help some of those who only partially respond to our existing antipsychotics.’
Precisely how Cobenfy works is not clear. By stimulating muscarinic receptors, it may ‘indirectly still have some of its effect by altering the dopamine system, but this is a very different mode of action,’ says McCutcheon.
‘We think different factors underlie negative symptoms that are not helped – and in fact potentially made worse – by blocking dopamine,’ he adds. ‘We are still working to understand how stimulating muscarinic receptors helps symptoms of psychosis. It may partly be by reducing dopamine release in that central bit of the brain but there are likely other mechanisms as well.’
In a statement from Bristol Myers Squibb, Rishi Kakar, an investigator in the clinical trials, noted that patients with schizophrenia often find themselves in a cycle of discontinuing and switching therapies. By operating on a different pathway, the drug ‘offers a new option to manage this changing condition,’ he said.